Abstract
Introduction
When a group of patients with Immune thrombocytopenia (ITP) (Bristol, UK) were asked to help prioritise research questions they identified glucocorticoid (GC) side effects, relapses and a trial and error approach to ITP treatment as the most impactful problems from their perspective. Although high dose GCs remain the most common first line treatment for adults with ITP and are recommended in guidelines, side effects are very common, with a survey reporting 98% had at least one side effect and 38% stopped or reduced dosage due to intolerable side effects (Brown et al, BMC Blood disorders, 2012; 12:2). Heterogeneous responses and high relapse rates are also significant problems; approximately 20% of patients with ITP are refractory to GCs, so-called steroid-resistant (SR) individuals, and only approximately 20% of patients maintain long term remission. There is currently no way to predict the success of GC treatment prior to administration. Consequently, there is an unmet need for a clinically validated biomarker of clinical GC responsiveness. This would help personalise treatments, ensuring GCs are only given to patients likely to benefit, whilst more efficacious treatments are given earlier to those expected to be SR.
Immune cells can be isolated from patients' peripheral blood and cultured in vitro to examine their responses to GCs. Different CD4+ T cell subsets have differential responses to GC treatment, and we have recently shown that CD4+ T cells expressing the cytokine interleukin (IL)-17 (so-called Th17 cells) are GC resistant (Schewitz-Bowers et al, PNAS, 2015, 112(30):4080-5). The expression of the anti-inflammatory cytokine IL-10 has previously been reported to be impaired in T cells from SR asthma patients (Xystrakis et al, J. Clin. Invest., 2006, 116:146-155), and in the most pro-inflammatory subtypes of Th17 cells (Ramesh et al, J. Exp. Med., 2014, 211(1):89-104). We therefore hypothesized that poor clinical response to GCs can be predicted by analyzing the expression of IL-17 and IL-10 in patients' CD4+ T cells exposed to GCs in vitro .
Methods
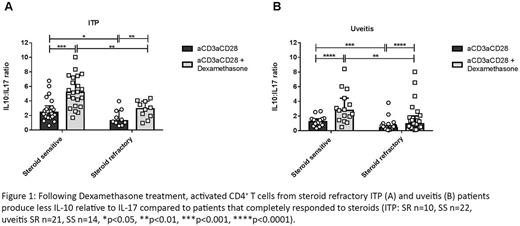
Following informed written consent, peripheral blood was drawn from patients with ITP requiring first line steroid treatment registered at University Hospitals Bristol, UK. Of these, 22 demonstrated complete response to steroid treatment with platelet count rising >100 x109/L within 2 weeks of treatment (steroid sensitive, SS) and 10 were refractory to treatment with a platelet count remaining <30x109/L following 2 weeks of treatment (steroid resistant, SR). Blood samples from patients with non-infectious intraocular inflammation (uveitis: an autoimmune condition treated with steroids) were also taken as a control disease cohort (SS n=14, SR n=21). CD4+ T cells were isolated and activated via the T cell receptor with anti-CD3/anti-CD28 T cell activation beads in the presence or absence of the synthetic GC Dexamethasone (Dex). After 96 hours of culture, intracellular cytokine expression of IL-10 and IL-17 was examined by multiparameter flow cytometry.
Results
CD4+ T cells isolated from clinically SR ITP patients showed higher expression of IL-17 and lower expression of IL-10, resulting in a reduced IL-10:IL-17 ratio when activated with anti-CD3/anti-CD28 beads in the presence of Dex (Figure 1A), compared to clinically SS patients (p=0.0015). Similar results were found in patients with uveitis (aCD3/aCD28+Dex SS vs SR p=0.0044), corroborating this finding (Figure 1B).
Conclusion
These data suggest that CD4+ T cells are important mediators of clinical response to steroid treatment and that the cytokine signature of CD4+ T cells may form the basis of a laboratory biomarker to help predict clinical response to GC treatment for patients with ITP as well as those with other autoimmune conditions. This would aid clinical decisions for patients, potentially maximizing treatment efficacy while minimizing side-effects.
Bradbury: Novartis: Consultancy, Other: Travel support; Amgen: Other: Conference attendence; BMS Pfizer: Consultancy, Honoraria, Speakers Bureau; Bayer: Other: Conference attendence. Lee: EMD Serono: Consultancy; University of Bristol: Patents & Royalties: International Patent Application No: PCT/GB2014/053804 / US Patent application No. 61/919,404 (A biomarker and companion therapeutic for steroid refractory diseases); Nanomerics: Consultancy; Roche: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal